杠杆配资公司_网上炒股配资_专业股票配资
你的位置:杠杆配资公司_网上炒股配资_专业股票配资 > 网上炒股配资 > 配资炒股行情 郑刚教授:降脂治疗与冠状动脉斑块消退
配资炒股行情 郑刚教授:降脂治疗与冠状动脉斑块消退
发布日期:2025-03-11 21:49 点击次数:87

低密度脂蛋白胆固醇(LDL-C)是动脉粥样硬化性心血管疾病(ASCVD)的关键致病因素。流行病学研究表明配资炒股行情,血浆LDL-C浓度与ASCVD风险之间存在剂量依赖关系[1]。除了LDL-C升高的幅度外,长期暴露于持续升高的LDL-C也被认为是个人ASCVD风险的主要因素[2]。
以LDL-C为靶点的降脂治疗是ASCVD预防的核心,其受益程度与强化他汀类药物单药治疗以及与依折麦布和前蛋白转化酶枯草杆菌蛋白酶9型(PCSK9)抑制剂联合使LDL-C降低的程度直接相关。降脂治疗对冠状动脉斑块体积和成分的有益影响被认为是降低心血管风险的关键机制。
在过去的几十年里,在许多临床试验中应用了几种侵入性和非侵入性成像技术,以评估降脂治疗对冠状动脉斑块负荷和局部斑块特征的影响。本文总结了既往获得的关于降脂治疗策略对动脉粥样硬化斑块消退影响的数据,这些数据主要通过几种成像方式评估。
斑块形成和进展的机制
斑块形成是一个复杂的、多因素的、多步骤的过程,通常持续数年或数十年。特别是,在持续暴露于多种致病风险因素(如高血压、血脂异常、糖尿病和吸烟)导致的内皮功能障碍的情况下,LDL-C等脂蛋白颗粒穿过内皮屏障并留在动脉壁[4-6]。氧化应激诱导的炎性细胞募集促进巨噬细胞对修饰脂蛋白颗粒的摄取,形成了泡沫细胞。充满脂质的巨噬细胞和平滑肌细胞增殖产生的泡沫细胞的积聚导致纤维帽形成和斑块生长。炎性细胞浸润、平滑肌细胞死亡和基质降解导致易损斑块,斑块具有薄纤维帽和富含脂质的坏死核心。
动脉粥样硬化通常被认为是一种慢性进行性疾病,可能因多种缺血事件而变得复杂。动脉粥样硬化斑块总负荷的大小可能与循环LDL-C和其他含载脂蛋白B(ApoB)的浓度以及接触这些脂蛋白的总持续时间成正比[2]。斑块进展分为以下几个阶段:亚临床动脉粥样硬化(内膜增厚和内膜脂质条纹)、可能导致血栓形成事件的易损斑块和钙化的稳定斑块。易损斑块的特征通常是斑块负荷增加、正向重塑、薄纤维帽覆盖的大脂质核心、巨噬细胞积聚和新生血管[7]。
斑块消退及其对临床结果的影响
斑块消退传统上被定义为冠状动脉造影管腔直径的增加,作为斑块尺寸减小的替代标志[8]。然而,斑块成像技术的最新进展不仅可以直接评估动脉粥样硬化斑块负荷,还可以评估斑块成分。除了使用血管内超声(IVUS)对未来心血管事件进行替代测量的斑块负荷外,斑块成分也是斑块相关缺血性事件的相关决定因素。在这种情况下,斑块消退可以定义为斑块形态的有利变化,包括动脉粥样硬化体积和降低随后心血管事件风险的成分[9]。在日常实践中,在强化一级/二级预防后,通常会观察到斑块消退,其特征是斑块体积减少,从不稳定斑块变为稳定钙化斑块。
斑块消退的临床相关性已在之前的荟萃分析中得到解决[10-11]。最近的一项荟萃回归分析综合了23项降脂治疗研究,报告了IVUS动脉粥样硬化体积百分比和临床结果的变化,表明斑块消退与心血管事件减少之间存在显著关联[12]。在7 407名试验持续时间为11~104周的患者中,一项调整后的分析显示平均动脉粥样硬化体积百分比(PAV)降低1%与主要不良心血管终点(MACE)的几率降低14%相关(调整后的比值比[HR]=0.86)。这些结果表明,PAV的变化可能是心血管事件的替代标志物,因此可能突出冠状动脉斑块时间评估的临床相关性,以更准确地指导降脂治疗的滴定决策。
通过影像学方式评估斑块
在之前的临床研究中,已经使用了几种侵入性和非侵入性成像方式来评估斑块负荷和成分。图1总结了用于评估冠状动脉斑块的每种成像模式的特征。
01 侵入性成像
IVUS成像基于换能器振荡运动产生的超声波,并利用各种动脉结构的反射差异来生成动脉壁和管腔的横截面图像。IVUS的分辨率为100~150µm,穿透深度为4~8mm。通过检测管腔和血管轮廓,IVUS可以提供斑块负荷(斑块面积=血管面积-管腔面积)。因此,血管内超声一直是评估动脉粥样硬化斑块的主要侵入性方法,目前被认为是斑块定量的金标准。
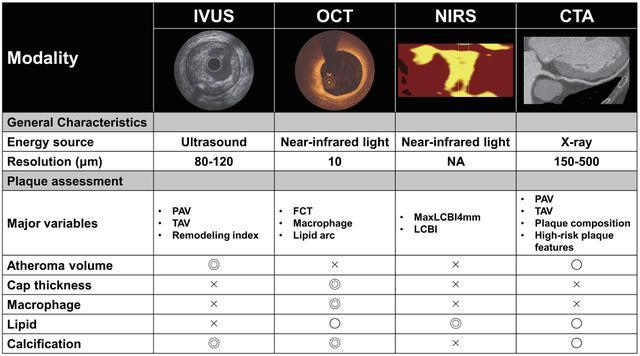
图1.评估冠状动脉斑块的影像学特征。
CTA,计算机断层摄影血管造影;FCT,纤维帽厚度;IVUS,血管内超声;LCBI,脂质核心负荷指数;NA,不可用;NIRS,近红外光谱;OCT,光学相干断层扫描;PAV,动脉粥样硬化体积百分比;TAV,动脉粥样硬化总体积。
由于分辨率有限,IVUS无法可靠地评估纤维帽厚度和详细的斑块成分。之前的一项使用计算机断层扫描血管造影(CTA)数据的研究报告称,与动脉粥样硬化总体积(TAV)和标准化TAV相比,PAV受体表面积的影响较小,可能是量化冠状动脉粥样硬化负担的最佳变量[13]。使用射频背向散射数据的光谱分析,如虚拟组织学(VH)-IVUS、背向散射积分(IB)-IWUS和iMAP,通过几种后处理方法评估了斑块成分。VH-IVUS是以前最常用的斑块消退评估方法之一,并可提供斑块成分信息,包括坏死核心、致密钙、纤维和纤维脂肪组织[14]。尽管VH-IVUS衍生的薄帽纤维粥样斑块(TCFA)已被用作未来心血管事件的高危特征[15],但据报道,由于其分辨率有限,诊断能力低于光学相干断层扫描(OCT)[16]。因此,这些模式在当前的日常实践中已不再使用。
OCT成像使用近红外光获得,较IVUS的分辨率(10~20μm)高,可提供更详细的浅表动脉壁微观结构图像,包括纤维帽厚度、巨噬细胞、溃疡和血栓。OCT衍生的TCFA是一种易发生急性冠状动脉事件的高危斑块特征,通常定义为纤维帽厚度<65μm[17]。OCT得出的主要结果指标包括最小纤维帽厚度(FCT)、巨噬细胞角和脂质弧。然而,由于组织渗透性低(即最大2mm),OCT不能准确测量斑块负荷和血管大小,特别是在脂质组织中。近红外光谱(NIRS)使用背散射光的光谱分析,提供动脉壁中胆固醇含量的信息。将NIRS与IVUS相结合有助于进行准确和客观的评估(即减少操作员依赖性)富含脂质的斑块,管腔和斑块可见。脂质的量被描述为脂质核心负荷指数(LCBI),通过将黄色像素的数量除以可用像素的总数,再乘以1000(范围从0~1000)来计算[18]。maxLCBI4mm通常用于量化介入靶区内脂质斑块的最大区域,分为4mm的冠状动脉段。之前的一项影像学研究报告称,NIRS衍生的富含脂质的斑块与IVUS和OCT上的高危斑块特征密切相关[19]。
02 非侵入性成像技术
冠状动脉CTA是一种非侵入性成像技术,可以定量评估冠状动脉狭窄和斑块。CTA根据Hounsfield单位将斑块类型分为低衰减、纤维脂肪、纤维和钙化斑块[20]。CTA检测到的高危斑块的定性特征包括低衰减斑块、阳性重塑、斑点状钙化和餐巾环征[21]。与仅能显示成像段的冠状动脉内成像相比,CTA可以提供完整的冠状动脉树信息,包括狭窄、斑块体积和斑块成分。最近,CTA已被证明可以显示冠状动脉周围脂肪组织的衰减,这反映了冠状动脉炎症的程度[22]。CTA的局限性包括比冠状动脉内成像和成像伪影更低的分辨率,如运动和束硬化,这可能会导致斑块成分的错误分类。
其他非侵入性技术包括心脏磁共振(CMR)和正电子发射断层扫描(PET)。CMR可以检测冠状动脉狭窄并表征血管壁,包括阳性重塑[23]。然而,由于其有限的空间分辨率和耗时的性质,CMR在日常实践中并不常用于冠状动脉评估,也没有研究检查冠状动脉斑块消退。PET可以使用放射性配体(例如18F-氟脱氧葡萄糖[18F-FDG]和18F-氟化钠)[24]。由于心肌摄取18F-FDG,评估冠状动脉斑块炎症的分辨率有限。与血管微钙化结合的新型放射性示踪剂18F-氟化钠在识别罪犯病变和易损斑块方面显示出良好的诊断能力,尤其是当与CT或CMR结合时[25-26]。
降脂治疗对冠状动脉斑块的影响
许多影像学研究评估了降脂治疗对冠状动脉斑块的影响,并为降脂治疗的心血管益处提供了机制基础。图2总结了先前药理学试验(他汀类药物、依折麦布和PCSK9抑制剂)中LDL-C水平与斑块消退之间的关系。尽管根据研究设计(如基线脂质管理、随访时间、患者表现、基线斑块负荷)和所调查的药物,各研究之间存在很大差异,但在之前的随机试验中,PAV(-5.7%至+0.9%)、TAV(-26.1%至+0.2%)、minFCT(+7.7μm至+110.1μm)和maxLCBI4mm(-149.1至-5.2)的有利变化得到了对照试验(RCTs)和观察性研究(图2)的证实。
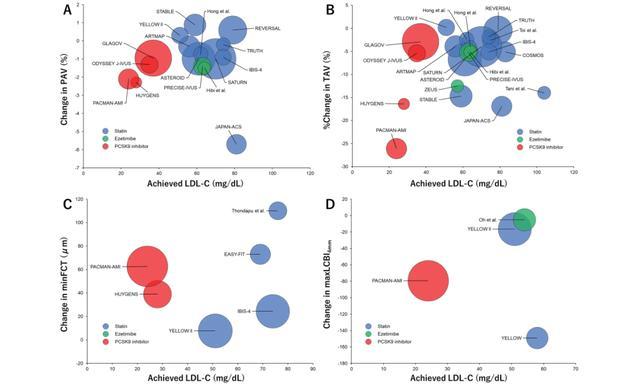
图2冠状动脉内成像斑块变量与LDL-C之间的相关性
气泡图显示了PAV(A)的变化、TAV(B)的变化百分比、最小FCT(C)的变化和最大LCBI4mm(D)的变化(y轴),与主要斑块消退试验的治疗组中达到的LDL-C(x轴)相比(未显示对照组)。气泡大小与治疗组中的患者数量成正比。FCT,纤维帽厚度;LCBI,脂质核心负荷指数;LDL-C,低密度脂蛋白胆固醇;PAV,动脉粥样硬化体积百分比;TAV,动脉粥样硬化总体积。
01 他汀类药物
他汀类药物通过抑制HMG-CoA还原酶来减少肝脏中的胆固醇合成。细胞内胆固醇的减少促进了肝细胞表面LDL受体的表达,导致血液中LDL-C的摄取增加,LDL和其他含ApoB的脂蛋白(包括富含甘油三酯(TG)的颗粒)的血浆浓度降低。尽管LDL-C的降低程度呈剂量依赖性,且LDL-C降低存在相当大的个体间差异,但他汀类药物通常会将LDL-C水平降低约30%~50%[27]。
几项研究调查了他汀类药物对冠状动脉斑块的影响。这些研究大多使用IVUS来评估斑块负荷的变化,VH-IVUS、OCT和NIRS已被用于评估斑块成分。斑块消退的程度与LDL-C降低的程度相关,类似于缺血风险降低与血清LDL-C水平之间的关联[28]。1997年,Takagi等[29]首次报道了他汀类药物对冠状动脉斑块的有益作用,如IVUS所评估的。与仅接受饮食稳定治疗的患者(n=12)相比,接受普伐他汀10 mg治疗的患者在36个月时斑块面积显著减少(PAV:-7±23 vs +41±23,P<0.0005)。从那时起,进行了很多临床试验来探究降脂治疗对冠状动脉斑块的影响。既往的一些随机对照试验显示,与低强度他汀类药相比,高强度他汀类药物可以诱导更大的斑块体积减少。2004年的REVERSAL试验报告称,在654名冠状动脉疾病(CAD)患者中,阿托伐他汀80mg与普伐他汀40mg相比,在18个月时通过IVUS减少了冠状动脉粥样硬化的进展(TAV变化百分比:-0.4% vs +2.7%;P=0.02)[30]。2011年的SATURN试验在1039名CAD患者中比较了阿托伐他汀80 mg和瑞舒伐他汀40 mg,并报告称,这两种方案在24个月时均显著降低了PAV(-0.99% vs -1.22%,P=0.17)[31]。2009年的急性冠状动脉综合征(ACS)试验报告称,在307名ACS患者中,匹伐他汀4 mg和阿托伐他汀20 mg在9个月时同样导致冠状动脉斑块体积显著下降(TAV:-5.7% vs -6.3%、P=0.5)[32]。2016年横滨ACS研究调查了4种不同他汀类药物对118名ACS患者的冠状动脉斑块。在10个月时,阿托伐他汀20 mg和匹伐他汀4 mg组的斑块体积减少幅度大于普伐他汀10 mg和氟伐他汀30 mg组(TAV:-3.6% vs -2.9% vs +1.5% vs +0.4%,P=0.02)[33]。
他汀类药物对斑块组成的影响已在几项使用VH-IVUS、IB-IVUS,OCT和NIRS的研究中进行了评估。VH-和IB-IVUS研究通常表明斑块稳定,包括纤维和钙化斑块体积增加,坏死核心和非钙化斑块体积减少。在OCT试验中,2014年EASY-FIT试验调查了70名不稳定型心绞痛患者中阿托伐他汀20 mg与阿托伐他汀5 mg对斑块稳定的影响。在1年时,阿托伐他汀20 mg显著提高了最小FCT(+73μm vs +19μm,P=0.002),降低了脂质弧度(-50°vs -10°,P<0.001)[34]。2018年的IBIS-4观察性研究表明,在103名ST段抬高型心肌梗死(STEMI)患者中,瑞舒伐他汀40 mg在1年内显著增加了最小FCT(+24μm),减少了巨噬细胞弧(-3°)。使用近红外光谱黄色试验表明,与标准治疗组相比,瑞舒伐他汀40 mg组在7周时最大LCBI4mm显著降低(-149 vs +2.4,P=0.01)[36]。几项CTA研究表明,他汀类降脂治疗可以减轻斑块体积进展[37-39]。2018年的PARADIGM研究调查了他汀类药物对1255名无冠心病(CAD)病史患者的冠状动脉斑块的影响。与他汀类药物患者的病变相比,他汀类药物服用患者的总体PAV进展速度较慢(每年1.76%±2.40% vs每年2.04%±2.37%,P=0.002),但钙化PAV进展更快(每年1.27%±1.54% vs 每年0.98%±1.27%,P<0.001)[38]。
02 依折麦布
依折麦布可抑制肠道对膳食和胆汁胆固醇的摄取,而不影响脂溶性营养素的吸收,从而减少输送到肝脏的胆固醇量。由于胆固醇输送减少,肝细胞表面LDL受体表达上调,导致血液中LDL-C的清除率增加。当添加到正在进行的他汀类药物治疗中时,依折麦布在有或没有CAD的血脂异常患者中可将LDL-C水平额外降低21%~27%[27]。
几项使用IVUS的随机试验评估了除他汀类药物外,依折麦布10mg对冠状动脉斑块消退的益处,并一致显示斑块消退(PAV变化)范围为-0.4%~-2.9%。2012年的HEAVEN试验表明[40],在89名CAD稳定的患者中,与标准治疗(即单独使用他汀类药物)相比,依折麦布10mg和阿托伐他汀80mg在1年时显著降低了PAV(-0.4% vs +1.4,P=0.014)。Masuda等[41]在2015年证明,依折麦布联合瑞舒伐他汀5 mg在6个月时显著降低了TAV(-13.2% vs -3.1%; P=0.05)与单独使用瑞舒伐他汀5mg治疗51名CAD稳定的患者相比。2015年的PRECISE-IVUS同样显示,在202名接受经皮冠状动脉介入治疗(PCI)的患者中,与单独使用阿托伐他汀相比,阿托伐他汀联合依折麦布可使斑块明显消退(PAV变化:-1.4% vs -0.3%,P=0.001)[42]。其他试验,包括ZEUS(2014)[43]和OCTIVUS(2016)[44]试验以及Hibi等[45](2018)和Oh等[46](2021)的研究,显示依折麦布联合他汀类药物治疗的患者斑块消退程度在数值上比单独使用他汀类药物的患者更大;然而,这些差异在统计学上并不显著。使用VH-IVUS[40-44]、IB-IVUS[45]和NIRS-IVUS[46]评估依折麦布与他汀类药物治疗对斑块组成的影响,但治疗组之间没有观察到显著差异。
2017年的ZIPANGU研究报告称,在131名稳定型CAD患者中,联合治疗组(阿托伐他汀10~20mg加依折麦布)和单药治疗组(阿伐他汀10~20mg)的血管镜黄色等级(等级越高,表明脂质越多)显著降低,但治疗组之间没有显著差异[47]。PCSK9抑制剂血浆中PCSK9浓度或功能的增加通过促进溶酶体分解代谢来降低LDL受体表达,导致血浆LDL浓度增加。
03 PCSK9抑制剂
主要使用针对PCSK9的人源化单克隆抗体开发了治疗策略,该抗体特异性结合PCSK9以抑制其对LDL受体的影响,导致LDL-C降低高达60%。英克司兰(Inclisiran)是一种新型的基于小干扰RNA的疗法,可抑制PCSK9的合成,使LDL-C降低高达50%[27];然而,目前关于其对斑块消退影响的证据仅限于抗人PCSK9单克隆抗体。2016年的GLAGOV试验通过IVUS 48评估了依洛尤单抗(evolocumab)和他汀类药物对冠状动脉斑块的影响。在968名接受冠状动脉造影的患者中,与安慰剂组相比,依洛尤单抗组在76周时的PAV显著降低(-0.95% vs +0.05%,P<0.01)。2019年的ODYSSEY J-IVUS试验使用IVUS评估了206名近期ACS患者服用75 mg阿利西尤单抗对冠状动脉斑块的影响。36周时,阿利西尤单抗组和标准治疗组的标准化TAV变化百分比没有显著差异(-4.8% vs -3.1%,P=0.23)。2022年的PACMANAMI试验使用NIRS-IVUS和OCT[50]和其他多效性效应[51-53]。在300名AMI患者中研究了在他汀类药物治疗中添加阿利西尤单抗对冠状动脉粥样硬化的影响。1年时,阿利西尤单抗导致的斑块体积减少较安慰剂更大(PAV变化:-2.13%vs -0.92%,P<0.001)。此外,阿利西尤单抗对斑块成分有良好的影响(近红外光谱显示最大LCBI4mm:-79.42 vs -37.60,P=0.006,OCT显示最小FCT:62.67μm vs 33.19μm,P<0.001)[54]。
PACMAN-AMI研究的一项子研究报告称,阿利西尤单抗治疗和较高的基线最大LCBI4mm是“三重逆转”的独立预测因素(即PAV减少、最大LCBI4mm减少和最小FCT增加),这与MACE风险降低有关[55]。同样,2022年的HUYGENS试验报告称,在161名非STEMI患者中,依洛尤单抗在1年时较安慰剂导致最小FCT增加更多(+42.7 vs +21.5μm,P=0.015)。
04 降低甘油三酯药物
众所周知,高TG水平(>150mg/dL)是潜在的CVD危险因素,但目前的指南建议,只有当TG>200 mg/dL且仅通过生活方式措施无法充分降低时,才考虑在高危患者中使用药物降低TG水平[27]。几项研究使用IVUS、OCT和CTA研究了二十碳五烯酸(EPA)对冠状动脉斑块的影响,结果喜忧参半。2017年的CHERRY研究报告称,在193名接受PCI的患者中,与单独使用匹伐他汀4mg相比,匹伐他汀4 mg和EPA 1800 mg/天的组合在8个月时显著降低了IB-IVUS引起的TAV。2020年的EVAPORATE试验表明,在18个月时,二十碳五烯酸乙酯(icosapent ethyl,IPE)4 g/天组的CTA斑块体积变化百分比较安慰剂组(-9%比+11%,P=0.0019)显著降低。然而,需要仔细考虑IPE组的高基线斑块体积和使用矿物油作为安慰剂,这对炎症和脂质状况有不利影响[58]。另外两项使用IB-IVUS(Niki等[59],2016)和CTA(Alfaddagh等[60],2017)的随机对照试验显示,斑块体积的变化没有显著差异。Nishio等[61]在2014年报告称,在30名接受PCI的患者中,与单独使用瑞舒伐他汀相比,在9个月时,在瑞舒伐他丁中添加EPA 1800 mg/天显著增加了OCT的FCT(+54.8μm vs +23.5μm,P<0.001),而Kita等[62]在2020年报告说,除他汀类药物治疗外,EPA或EPA+二十二碳六烯酸(DHA)治疗并没有显著增加FCT。
迄今为止,还没有关于贝特类药物对冠状动脉斑块消退影响的研究报告。目前,一项随机试验正在进行中,该试验调查了培马贝特对CAD和空腹TG水平升高患者的冠状动脉斑块和肾功能的影响(PEMA-CORE研究)。
05 与斑块消退迟钝相关的因素
多项研究一致报告称,糖尿病的存在与降脂治疗使冠状动脉斑块消退迟钝有关[67-68]。之前对5项随机对照试验(包括2237名接受连续IVUS成像以评估几种药物对冠状动脉斑块进展影响的患者)的荟萃分析表明,糖尿病患者的斑块进展比非糖尿病患者更大(PAV:+0.6% vs +0.05%,P=0.0001,TAV:-0.6% vs -2.7%,P=0.03)[63]。糖尿病的存在可能会减弱降脂治疗对斑块消退的影响。
脂蛋白(a)(Lp[a])对斑块消退的影响存在争议。YOKOHAMA-ACS研究的一个子分析报告称,与Lp(a)≤20mg/dl的患者相比,Lp(a)>20mg/dl患者的斑块消退减弱(PAV:+2.5% vs -6.8%,p=0.02)[68],而SATURN研究的一个子分析表明,Lp(a)水平与PAV的变化无关[69]。
总结和未来展望
几种成像技术已应用于临床试验,以确定降脂治疗对冠状动脉斑块负荷和成分的影响。尽管IVUS是评估斑块负荷最广泛使用的方法,但其他侵入性方法,如OCT和近红外光谱,可以提供斑块易损性的相关数据,而CTA可以非侵入性地检测斑块体积和特征。大量证据支持他汀类药物单独或与依折麦布和PCSK9抑制剂联合降低LDL-C持续改善斑块负荷和有利的形态学变化的观点。未来的研究应侧重于新的治疗方案对新兴治疗靶点的影响,包括Lp(a)、TG和炎症对冠状动脉斑块的影响,尽管需要明确的临床终点试验来确认临床疗效和潜在的不良反应。此外,鉴于斑块消退与心血管事件减少之间的因果关系,直接进行斑块成像以监测动脉粥样硬化斑块体积和成分的时间变化可能在指导一级和二级预防的治疗决策方面发挥一定的作用。常规斑块成像指导下的最佳药物治疗是否真的能改善临床结果,仍需进一步研究证实。
参考文献:
1) Ference BA, Ginsberg HN, Graham I, Ray KK, Packard CJ, Bruckert E, Hegele RA, Krauss RM, Raal FJ,Schunkert H, Watts GF, Borén J, Fazio S, Horton JD,Masana L, Nicholls SJ, Nordestgaard BG, van de Sluis B,Taskinen M-R, Tokgözoğlu L, Landmesser U, Laufs U,Wiklund O, Stock JK, Chapman MJ, Catapano AL. Low-density lipoproteins cause atherosclerotic cardiovascular
disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European
Atherosclerosis Society Consensus Panel. European Heart Journal, 2017; 38: 2459-2472
2) Ference BA, Graham I, Tokgozoglu L, Catapano AL.Impact of Lipids on Cardiovascular Health. Journal of the American College of Cardiology, 2018; 72: 1141-1156
3) Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Barnes EH, Keech A, Simes J,Collins R. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet, 2010; 376:1670-1681
4) Libby P, Buring JE, Badimon L, Hansson GK, Deanfield J, Bittencourt MS, Tokgözoğlu L, Lewis EF.Atherosclerosis. Nat Rev Dis Primers, 2019; 5: 56
5) Libby P. The changing landscape of atherosclerosis.Nature, 2021; 592: 524-533
6) Stone PH, Libby P, Boden WE. Fundamental Pathobiology of Coronary Atherosclerosis and Clinical Implications for Chronic Ischemic Heart Disease Management-The Plaque Hypothesis: A Narrative Review. JAMA Cardiol, 2023; 8: 192-201
7) Virmani R, Burke AP, Farb A, Kolodgie FD. Pathology of the vulnerable plaque. J Am Coll Cardiol, 2006; 47: C13-18
8) Ahmadi A, Argulian E, Leipsic J, Newby DE, Narula J.From Subclinical Atherosclerosis to Plaque Progression and Acute Coronary Events. Journal of the American College of Cardiology, 2019; 74: 1608-1617
9) Dawson LP, Lum M, Nerleker N, Nicholls SJ, Layland J.Coronary Atherosclerotic Plaque Regression: JACC State-of-the-Art Review. J Am Coll Cardiol, 2022; 79: 66-82
10) Bhindi R, Guan M, Zhao Y, Humphries KH, Mancini GBJ. Coronary atheroma regression and adverse cardiac events: A systematic review and meta-regression analysis.Atherosclerosis, 2019; 284: 194-201
11) Nicholls SJ, Hsu A, Wolski K, Hu B, Bayturan O, Lavoie A, Uno K, Tuzcu EM, Nissen SE. Intravascular ultrasound-derived measures of coronary atherosclerotic plaque burden and clinical outcome. J Am Coll Cardiol,2010; 55: 2399-2407
12) Iatan I, Guan M, Humphries KH, Yeoh E, Mancini GBJ.Atherosclerotic Coronary Plaque Regression and Risk of Adverse Cardiovascular Events: A Systematic Review and Updated Meta-Regression Analysis. JAMA Cardiol, 2023;8: 937-945
13) van Rosendael AR, Lin FY, Ma X, van den Hoogen IJ,Gianni U, Al Hussein O, Al’Aref SJ, Peña JM, Andreini D, Al-Mallah MH, Budoff MJ, Cademartiri F,Chinnaiyan K, Choi JH, Conte E, Marques H, de Araújo Gonçalves P, Gottlieb I, Hadamitzky M, Leipsic JA,Maffei E, Pontone G, Raff GL, Shin S, Kim Y-J, Lee BK,Chun EJ, Sung JM, Lee S-E, Berman DS, Virmani R,Samady H, Stone PH, Narula J, Bax JJ, Shaw LJ, Min JK,Chang H-J. Percent atheroma volume: Optimal variable to report whole-heart atherosclerotic plaque burden with coronary CTA, the PARADIGM study. Journal of Cardiovascular Computed Tomography, 2020; 14: 400-
406
14) Garcia-Garcia HM, Costa MA, Serruys PW. Imaging of coronary atherosclerosis: intravascular ultrasound. Eur Heart J, 2010; 31: 2456-2469
15) Stone GW, Maehara A, Lansky AJ, de Bruyne B, Cristea E, Mintz GS, Mehran R, McPherson J, Farhat N, Marso SP, Parise H, Templin B, White R, Zhang Z, Serruys PW.A prospective natural-history study of coronary atherosclerosis. N Engl J Med, 2011; 364: 226-235
16) Ueki Y, Yamaji K, Losdat S, Karagiannis A, Taniwaki M,Roffi M, Otsuka T, Koskinas KC, Holmvang L,Maldonado R, Pedrazzini G, Radu MD, Dijkstra J,Windecker S, Garcia-Garcia HM, Raber L. Discordance in the diagnostic assessment of vulnerable plaques between radiofrequency intravascular ultrasound versus optical coherence tomography among patients with acute myocardial infarction: insights from the IBIS-4 study. Int J Cardiovasc Imaging, 2021; 37: 2839-2847
17) Narula J, Nakano M, Virmani R, Kolodgie FD, Petersen R, Newcomb R, Malik S, Fuster V, Finn AV.Histopathologic characteristics of atherosclerotic coronary disease and implications of the findings for the invasive and noninvasive detection of vulnerable plaques. J Am Coll Cardiol, 2013; 61: 1041-1051
18) Johnson TW, Raber L, di Mario C, Bourantas C, Jia H,Mattesini A, Gonzalo N, de la Torre Hernandez JM, Prati F, Koskinas K, Joner M, Radu MD, Erlinge D, Regar E, Kunadian V, Maehara A, Byrne RA, Capodanno D,Akasaka T, Wijns W, Mintz GS, Guagliumi G. Clinical use of intracoronary imaging. Part 2: acute coronary syndromes, ambiguous coronary angiography findings,and guiding interventional decision-making: an expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. Eur Heart J,2019; 40: 2566-2584
19) Zanchin C, Ueki Y, Losdat S, Fahrni G, Daemen J,Ondracek AS, Haner JD, Stortecky S, Otsuka T, Siontis GCM, Rigamonti F, Radu M, Spirk D, Kaiser C,Engstrom T, Lang I, Koskinas KC, Raber L. In vivo relationship between near-infrared spectroscopy
-detectedipid-rich plaques and morphological plaque characteristics by optical coherence tomography and
intravascular ultrasound: a multimodality intravascular imaging study. Eur Heart J Cardiovasc Imaging, 2020;
20) Pundziute G, Schuijf JD, Jukema JW, Decramer I, Sarno G, Vanhoenacker PK, Boersma E, Reiber JHC, Schalij MJ, Wijns W, Bax JJ. Evaluation of plaque characteristics in acute coronary syndromes: non-invasive assessment with multi-slice computed tomography and invasive evaluation with intravascular ultrasound radiofrequency data analysis. European Heart Journal, 2008; 29: 2373-2381
21) Motoyama S, Ito H, Sarai M, Kondo T, Kawai H,Nagahara Y, Harigaya H, Kan S, Anno H, Takahashi H,Naruse H, Ishii J, Hecht H, Shaw LJ, Ozaki Y, Narula J.Plaque Characterization by Coronary Computed Tomography Angiography and the Likelihood of Acute Coronary Events in Mid-Term Follow-Up. J Am Coll Cardiol, 2015; 66: 337-346
22) Tan N, Dey D, Marwick TH, Nerlekar N. Pericoronary Adipose Tissue as a Marker of Cardiovascular Risk: JACC Review Topic of the Week. J Am Coll Cardiol, 2023; 81:913-923
23) He Y, Zhang Z, Dai Q, Zhou Y, Yang Y, Yu W, An J, Jin L, Jerecic R, Yuan C, Li D. Accuracy of MRI to identify the coronary artery plaque: A comparative study with intravascular ultrasound. Journal of Magnetic Resonance Imaging, 2011; 35: 72-78
24) Evans NR, Tarkin JM, Chowdhury MM, Warburton EA,Rudd JHF. PET Imaging of Atherosclerotic Disease:Advancing Plaque Assessment from Anatomy to Pathophysiology. Current Atherosclerosis Reports, 2016;18:
25) Moss AJ, Doris MK, Andrews JPM, Bing R, Daghem M,van Beek EJR, Forsyth L, Shah ASV, Williams MC,Sellers S, Leipsic J, Dweck MR, Parker RA, Newby DE,Adamson PD. Molecular Coronary Plaque Imaging Using 18 F-Fluoride. Circulation: Cardiovascular Imaging,2019; 12:
26) Joshi NV, Vesey AT, Williams MC, Shah AS, Calvert PA,Craighead FH, Yeoh SE, Wallace W, Salter D, Fletcher AM, van Beek EJ, Flapan AD, Uren NG, Behan MW,Cruden NL, Mills NL, Fox KA, Rudd JH, Dweck MR,Newby DE. 18F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: a prospective clinical trial. Lancet,2014; 383: 705-713
27) Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman MJ, De Backer GG, Delgado V, Ference BA, Graham IM, Halliday A, Landmesser U,Mihaylova B, Pedersen TR, Riccardi G, Richter DJ,Sabatine MS, Taskinen MR, Tokgozoglu L, Wiklund O,Group ESCSD. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J, 2019;
28) Li Y, Deng S, Liu B, Yan Y, Du J, Li Y, Jing X, Liu Y,Wang J, Du J, She Q. The effects of lipid-lowering therapy on coronary plaque regression: a systematic review and meta-analysis. Scientific Reports, 2021; 11:
29) Takagi T, Yoshida K, Akasaka T, Hozumi T, Morioka S,Yoshikawa J. Intravascular ultrasound analysis ofreduction in progression of coronary narrowing by treatment with pravastatin. Am J Cardiol, 1997; 79:1673-1676
30) Nissen SE, Tuzcu EM, Schoenhagen P, Brown BG, Ganz P, Vogel RA, Crowe T, Howard G, Cooper CJ, Brodie B,Grines CL, DeMaria AN. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial.Jama, 2004; 291: 1071-1080
31) Nicholls SJ, Ballantyne CM, Barter PJ, Chapman MJ,Erbel RM, Libby P, Raichlen JS, Uno K, Borgman M,Wolski K, Nissen SE. Effect of two intensive statin regimens on progression of coronary disease. N Engl J Med, 2011; 365: 2078-2087
32) Hiro T, Kimura T, Morimoto T, Miyauchi K, Nakagawa Y, Yamagishi M, Ozaki Y, Kimura K, Saito S, Yamaguchi T, Daida H, Matsuzaki M, Investigators J-A. Effect of intensive statin therapy on regression of coronary atherosclerosis in patients with acute coronary syndrome:a multicenter randomized trial evaluated by volumetric intravascular ultrasound using pitavastatin versus atorvastatin (JAPAN-ACS [Japan assessment of pitavastatin and atorvastatin in acute coronary syndrome] study). J Am Coll Cardiol, 2009; 54: 293-302
33) Matsushita K, Hibi K, Komura N, Akiyama E, Maejima N, Iwahashi N, Tsukahara K, Kosuge M, Ebina T, Sumita S, Umemura S, Kimura K. Effects of 4 Statins on Regression of Coronary Plaque in Acute Coronary Syndrome. Circ J, 2016; 80: 1634-1643
34) Komukai K, Kubo T, Kitabata H, Matsuo Y, Ozaki Y,Takarada S, Okumoto Y, Shiono Y, Orii M, Shimamura K, Ueno S, Yamano T, Tanimoto T, Ino Y, Yamaguchi T,Kumiko H, Tanaka A, Imanishi T, Akagi H, Akasaka T.Effect of atorvastatin therapy on fibrous cap thickness in coronary atherosclerotic plaque as assessed by optical coherence tomography: the EASY-FIT study. J Am Coll Cardiol, 2014; 64: 2207-2217
35) Raber L, Koskinas KC, Yamaji K, Taniwaki M, Roffi M,Holmvang L, Garcia Garcia HM, Zanchin T, Maldonado R, Moschovitis A, Pedrazzini G, Zaugg S, Dijkstra J,Matter CM, Serruys PW, Luscher TF, Kelbaek H,Karagiannis A, Radu MD, Windecker S. Changes in Coronary Plaque Composition in Patients With Acute Myocardial Infarction Treated With High-Intensity Statin
Therapy (IBIS-4): A Serial Optical Coherence Tomography Study. JACC Cardiovasc Imaging, 2018;
36) Kini AS, Baber U, Kovacic JC, Limaye A, Ali ZA, SweenyJ, Maehara A, Mehran R, Dangas G, Mintz GS, Fuster V,Narula J, Sharma SK, Moreno PR. Changes in plaque lipid content after short-term intensive versus standard statin therapy: the YELLOW trial (reduction in yellow plaque by aggressive lipid-lowering therapy). J Am Coll Cardiol, 2013; 62: 21-29
37) Smit JM, van Rosendael AR, El Mahdiui M, Neglia D,Knuuti J, Saraste A, Buechel RR, Teresinska A, Pizzi MN,Roque A, Poddighe R, Mertens BJ, Caselli C, Rocchiccioli S, Parodi O, Pelosi G, Scholte AJ. Impact of Clinical Characteristics and Statins on Coronary Plaque Progression by Serial Computed Tomography Angiography. Circ Cardiovasc Imaging, 2020; 13:
e009750
38) Lee SE, Chang HJ, Sung JM, Park HB, Heo R, Rizvi A,Lin FY, Kumar A, Hadamitzky M, Kim YJ, Conte E,Andreini D, Pontone G, Budoff MJ, Gottlieb I, Lee BK,Chun EJ, Cademartiri F, Maffei E, Marques H, Leipsic JA, Shin S, Choi JH, Chinnaiyan K, Raff G, Virmani R,Samady H, Stone PH, Berman DS, Narula J, Shaw LJ,Bax JJ, Min JK. Effects of Statins on Coronary Atherosclerotic Plaques: The PARADIGM Study. JACCCardiovasc Imaging, 2018; 11: 1475-1484
39) Shin S, Park HB, Chang HJ, Arsanjani R, Min JK, Kim YJ, Lee BK, Choi JH, Hong GR, Chung N. Impact of Intensive LDL Cholesterol Lowering on Coronary Artery Atherosclerosis Progression: A Serial CT Angiography Study. JACC Cardiovasc Imaging, 2017; 10: 437-446
40) Kovarnik T, Mintz GS, Skalicka H, Kral A, Horak J,Skulec R, Uhrova J, Martasek P, Downe RW, Wahle A,Sonka M, Mrazek V, Aschermann M, Linhart A. Virtual histology evaluation of atherosclerosis regression during atorvastatin and ezetimibe administration: HEAVEN study. Circ J, 2012; 76: 176-183
41) Masuda J, Tanigawa T, Yamada T, Nishimura Y, Sasou T,Nakata T, Sawai T, Fujimoto N, Dohi K, Miyahara M,Nishikawa M, Nakamura M, Ito M. Effect of combination therapy of ezetimibe and rosuvastatin on regression of coronary atherosclerosis in patients with coronary artery disease. Int Heart J, 2015; 56: 278-285
42) Tsujita K, Sugiyama S, Sumida H, Shimomura H,Yamashita T, Yamanaga K, Komura N, Sakamoto K, Oka H, Nakao K, Nakamura S, Ishihara M, Matsui K, Sakaino N, Nakamura N, Yamamoto N, Koide S, Matsumura T,Fujimoto K, Tsunoda R, Morikami Y, Matsuyama K,Oshima S, Kaikita K, Hokimoto S, Ogawa H,Investigators P-I. Impact of Dual Lipid-Lowering Strategy With Ezetimibe and Atorvastatin on Coronary Plaque Regression in Patients With Percutaneous Coronary Intervention: The Multicenter Randomized Controlled PRECISE-IVUS Trial. J Am Coll Cardiol, 2015; 66: 495-507
43) Nakajima N, Miyauchi K, Yokoyama T, Ogita M,Miyazaki T, Tamura H, Nishino A, Yokoyama K, Okazaki S, Kurata T, Suwa S, Daida H. Effect of combination of ezetimibe and a statin on coronary plaque regression in patients with acute coronary syndrome. IJC Metabolic &Endocrine, 2014; 3: 8-13
44) Hougaard M, Hansen HS, Thayssen P, Antonsen L,Junker A, Veien K, Jensen LO. Influence of ezetimibe in addition to high-dose atorvastatin therapy on plaque composition in patients with ST-segment elevation myocardial infarction assessed by serial: Intravascular ultrasound with iMap: the OCTIVUS trial. Cardiovasc Revasc Med, 2017; 18: 110-117
45) Hibi K, Sonoda S, Kawasaki M, Otsuji Y, Murohara T,Ishii H, Sato K, Koshida R, Ozaki Y, Sata M, Morino Y,Miyamoto T, Amano T, Morita S, Kozuma K, Kimura K,Fujiwara H, Ezetimibe ACSI. Effects of Ezetimibe-Statin Combination Therapy on Coronary Atherosclerosis in Acute Coronary Syndrome. Circ J, 2018; 82: 757-766
46) Oh PC, Jang AY, Ha K, Kim M, Moon J, Suh SY, Lee K,Han SH, Kang WC. Effect of Atorvastatin (10 mg) and Ezetimibe (10 mg) Combination Compared to Atorvastatin (40 mg) Alone on Coronary Atherosclerosis. Am J Cardiol, 2021; 154: 22-28
47) Ueda Y, Hiro T, Hirayama A, Komatsu S, Matsuoka H,Takayama T, Ishihara M, Hayashi T, Saito S, Kodama K,Investigators Z. Effect of Ezetimibe on Stabilization and Regression of Intracoronary Plaque - The ZIPANGU Study. Circ J, 2017; 81: 1611-1619
48) Nicholls SJ, Puri R, Anderson T, Ballantyne CM, Cho L,Kastelein JJ, Koenig W, Somaratne R, Kassahun H, Yang , Wasserman SM, Scott R, Ungi I, Podolec J, Ophuis AO, Cornel JH, Borgman M, Brennan DM, Nissen SE.Effect of Evolocumab on Progression of Coronary Disease in Statin-Treated Patients: The GLAGOV Randomized Clinical Trial. JAMA, 2016; 316: 2373-2384
49) Ako J, Hibi K, Tsujita K, Hiro T, Morino Y, Kozuma K,Shinke T, Otake H, Uno K, Louie MJ, Takagi Y,Miyauchi K. Effect of Alirocumab on Coronary Atheroma Volume in Japanese Patients With Acute Coronary Syndrome - The ODYSSEY J-IVUS Trial. Circ J, 2019;83: 2025-2033
50) Zanchin C, Koskinas KC, Ueki Y, Losdat S, Haner JD,Bar S, Otsuka T, Inderkum A, Jensen MRJ, Lonborg J,Fahrni G, Ondracek AS, Daemen J, van Geuns RJ,Iglesias JF, Matter CM, Spirk D, Juni P, Mach F, Heg D,Engstrom T, Lang I, Windecker S, Raber L. Effects of the PCSK9 antibody alirocumab on coronary atherosclerosis in patients with acute myocardial infarction: a serial,
multivessel, intravascular ultrasound, near-infrared spectroscopy and optical coherence tomography imaging
study-Rationale and design of the PACMAN-AMI trial.Am Heart J, 2021; 238: 33-44
51) Ueki Y, Haner J, Losdat S, Gargiulo G, Shibutani H, Bar S, Otsuka T, Kavaliauskaite R, Mitter V, Temperli F, Spirk D, Stortecky S, Siontis G, Valgimigli M, Windecker S,Gutmann C, Koskinas KC, Mayr M, Raber L. Effect of Alirocumab Added to High-Intensity Statin on Platelet Reactivity and Non-coding RNAs in AMI Patients: A Substudy of the PACMAN-AMI Trial. Thromb Haemost,2023;
52) Bar S, Kavaliauskaite R, Otsuka T, Ueki Y, Haner JD,Siontis GCM, Stortecky S, Shibutani H, Temperli F,Kaiser C, Iglesias JF, Jan van Geuns R, Daemen J, Spirk D, Engstrom T, Lang I, Windecker S, Koskinas KC,Losdat S, Raber L. Impact of alirocumab on plaque regression and haemodynamics of non-culprit arteries in patients with acute myocardial infarction: a prespecified
substudy of the PACMAN-AMI trial. EuroIntervention,2023;
53) Rexhaj E, Bär S, Soria R, Ueki Y, Häner JD, Otsuka T,Kavaliauskaite R, Siontis GCM, Stortecky S, Shibutani H,Spirk D, Engstrøm T, Lang I, Morf L, Ambühl M,Windecker S, Losdat S, Koskinas KC, Räber L. Effects of alirocumab on endothelial function and coronary atherosclerosis in myocardial infarction: A PACMAN-AMI randomized clinical trial substudy. Atherosclerosis,2024; 117504
54) Räber L, Ueki Y, Otsuka T, Losdat S, Häner JD, Lonborg J, Fahrni G, Iglesias JF, van Geuns R-J, Ondracek AS,Radu Juul Jensen MD, Zanchin C, Stortecky S, Spirk D,Siontis GCM, Saleh L, Matter CM, Daemen J, Mach F,Heg D, Windecker S, Engstrøm T, Lang IM, Koskinas KC, Ambühl M, Bär S, Frenk A, Morf LU, Inderkum A, Leuthard S, Kavaliauskaite R, Rexhaj E, Shibutani H,
Mitter VR, Kaiser C, Mayr M, Eberli FR, O’Sullivan CJ,Templin C, von Eckardstein A, Ghandilyan A, Pawar R,Jonker H, Hofbauer T, Goliasch G, Bang L, Sørensen R,Tovar Forero MN, Degrauwe S, Ten Cate T. Effect of Alirocumab Added to High-Intensity Statin Therapy on Coronary Atherosclerosis in Patients With Acute Myocardial Infarction. Jama, 2022;
55) Biccire FG, Haner J, Losdat S, Ueki Y, Shibutani H,Otsuka T, Kakizaki R, Hofbauer TM, van Geuns RJ,Stortecky S, Siontis GC, Bar S, Lonborg J, Heg D, Kaiser C, Spirk D, Daemen J, Iglesias J, Windecker S, Engstrom T, Lang I, Koskinas KC, Raber L. Concomitant Coronary Atheroma Regression and Stabilization in Response to Lipid-Lowering Therapy. J Am Coll Cardiol, 2023;
56) Nicholls SJ, Kataoka Y, Nissen SE, Prati F, Windecker S,Puri R, Hucko T, Aradi D, Herrman JR, Hermanides RS,Wang B, Wang H, Butters J, Di Giovanni G, Jones S,Pompili G, Psaltis PJ. Effect of Evolocumab on Coronary Plaque Phenotype and Burden in Statin-Treated Patients Following Myocardial Infarction. JACC Cardiovasc Imaging, 2022; 15: 1308-1321
57) Watanabe T, Ando K, Daidoji H, Otaki Y, Sugawara S,Matsui M, Ikeno E, Hirono O, Miyawaki H, Yashiro Y,Nishiyama S, Arimoto T, Takahashi H, Shishido T,Miyashita T, Miyamoto T, Kubota I, investigators Cs. A randomized controlled trial of eicosapentaenoic acid in patients with coronary heart disease on statins. J Cardiol,2017; 70: 537-544
58) Budoff MJ, Bhatt DL, Kinninger A, Lakshmanan S,Muhlestein JB, Le VT, May HT, Shaikh K, Shekar C, Roy SK, Tayek J, Nelson JR. Effect of icosapent ethyl on progression of coronary atherosclerosis in patients with elevated triglycerides on statin therapy: final results of the EVAPORATE trial. Eur Heart J, 2020; 41: 3925-3932
59) Niki T, Wakatsuki T, Yamaguchi K, Taketani Y, Oeduka H, Kusunose K, Ise T, Iwase T, Yamada H, Soeki T, Sata M. Effects of the Addition of Eicosapentaenoic Acid to Strong Statin Therapy on Inflammatory Cytokines and Coronary Plaque Components Assessed by Integrated Backscatter Intravascular Ultrasound. Circ J, 2016; 80:450-460
60) Alfaddagh A, Elajami TK, Ashfaque H, Saleh M, Bistrian BR, Welty FK. Effect of Eicosapentaenoic and Docosahexaenoic Acids Added to Statin Therapy on Coronary Artery Plaque in Patients With Coronary Artery Disease: A Randomized Clinical Trial. J Am Heart Assoc,2017; 6:
61) Nishio R, Shinke T, Otake H, Nakagawa M, Nagoshi R,Inoue T, Kozuki A, Hariki H, Osue T, Taniguchi Y,Iwasaki M, Hiranuma N, Konishi A, Kinutani H, Shite J,Hirata K. Stabilizing effect of combined eicosapentaenoic acid and statin therapy on coronary thin-cap fibroatheroma. Atherosclerosis, 2014; 234: 114-119
62) Kita Y, Watanabe M, Kamon D, Ueda T, Soeda T,Okayama S, Ishigami K, Kawata H, Horii M, Inoue F, Doi N, Okura H, Uemura S, Saito Y. Effects of Fatty Acid Therapy in Addition to Strong Statin on Coronary Plaques in Acute Coronary Syndrome: An Optical Coherence Tomography Study. J Am Heart Assoc, 2020;9: e015593
63) Nicholls SJ, Tuzcu EM, Kalidindi S, Wolski K, MoonKW, Sipahi I, Schoenhagen P, Nissen SE. Effect of diabetes on progression of coronary atherosclerosis and arterial remodeling: a pooled analysis of 5 intravascular ultrasound trials. J Am Coll Cardiol, 2008; 52: 255-262
64) Hiro T, Kimura T, Morimoto T, Miyauchi K, Nakagawa Y, Yamagishi M, Ozaki Y, Kimura K, Saito S, Yamaguchi T, Daida H, Matsuzaki M, Investigators J-A. Diabetes mellitus is a major negative determinant of coronary plaque regression during statin therapy in patients with acute coronary syndrome--serial intravascular ultrasound observations from the Japan Assessment of Pitavastatin and Atorvastatin in Acute Coronary Syndrome Trial (the JAPAN-ACS Trial). Circ J, 2010; 74: 1165-1174
65) Daida H, Takayama T, Hiro T, Yamagishi M, Hirayama A,Saito S, Yamaguchi T, Matsuzaki M. High HbA1c levels correlate with reduced plaque regression during statin treatment in patients with stable coronary artery disease:results of the coronary atherosclerosis study measuring effects of rosuvastatin using intravascular ultrasound in Japanese subjects (COSMOS). Cardiovasc Diabetol,2012; 11: 87
66) Kovarnik T, Chen Z, Mintz GS, Wahle A, Bayerova K,Kral A, Chval M, Kopriva K, Lopez J, Sonka M, Linhart A. Plaque volume and plaque risk profile in diabetic vs.non-diabetic patients undergoing lipid-lowering therapy:a study based on 3D intravascular ultrasound and virtual histology. Cardiovasc Diabetol, 2017; 16: 156
67) Fujisue K, Yamanaga K, Nagamatsu S, Shimomura H,Yamashita T, Nakao K, Nakamura S, Ishihara M, Matsui K, Sakaino N, Miyazaki T, Yamamoto N, Koide S,Matsumura T, Fujimoto K, Tsunoda R, Morikami Y,Matsuyama K, Oshima S, Sakamoto K, Izumiya Y,Kaikita K, Hokimoto S, Ogawa H, Tsujita K. Effects of Statin Plus Ezetimibe on Coronary Plaques in Acute Coronary Syndrome Patients with Diabetes Mellitus:Sub-Analysis of PRECISE-IVUS Trial. J Atheroscler Thromb, 2021; 28: 181-193
68) Matsushita K, Hibi K, Komura N, Kimura Y, Matsuzawa Y, Konishi M, Maejima N, Iwahashi N, Kosuge M, Ebina T, Tamura K, Kimura K. Impact of serum lipoprotein (a) level on coronary plaque progression and cardiovascular events in statin-treated patients with acute coronary syndrome: a yokohama-acs substudy. J Cardiol, 2020; 76:66-72
69) Puri R, Ballantyne CM, Hoogeveen RC, Shao M, Barter P, Libby P, Chapman MJ, Erbel R配资炒股行情, Arsenault BJ, Raichlen JS, Nissen SE, Nicholls SJ. Lipoprotein(a) and coronary atheroma progression rates during long-term high-intensity statin therapy: Insights from SATURN.Atherosclerosis, 2017; 263: 137-144

